Overcoming Challenges in Bioanalytical Assay Development for Innovative Drug Modalities

Key Learnings
- Understanding the unique properties of innovative drug modalities is crucial in bioanalytical assay development
- Simple modifications to existing protocols can significantly improve assay performance
- Detailed intake processes and client communication are essential for developing effective strategies
The landscape of drug development is dynamic, and innovative drug modalities like exosomes can present unique challenges that require both expertise and adaptability in bioanalytical assay development. In this blog, we showcase how our team tackled these challenges with Drug B, an exosome-based therapy targeting T cells and natural killer cells for lymphoma treatment.
Understanding Drug B
Drug B is an exosome, a cell-derived vesicle, which expresses IL-12 and targets T cells and natural killer cells to treat lymphoma. The drug is administered intratumorally to maximize its effectiveness by concentrating exposure within the tumor microenvironment. This innovative approach aims to enhance localized immune response and minimize systemic side effects, making Drug B a promising candidate for targeted cancer therapy.
Initial Challenges
Despite intratumoral administration, Drug B needed to be quantified accurately in the serum to monitor systemic exposure. We decided to leverage a commercial kit from Meso Scale Discovery (MSD) designed to quantify human IL-12 in serum. We used the client-provided IL-12 concentration of Drug B and a recombinant human IL-12 (a kit control) to prepare plate controls. However, in initial experiments, QCs prepared with Drug B only recovered about 50% compared to the kit control (recombinant human IL-12), which showed acceptable performance.
Problem-Solving Approach
Our team hypothesized that the size and structure of the exosome inhibited its ability to be captured effectively in the assay. We explored the use of Triton X-100, a common lysis detergent, to pretreat the samples and lyse the exosome, making more IL-12 available for detection.
Solution and Results
By incorporating a 0.5% Triton X-100 solution in the assay diluent, we saw significant improvements. The detergent did not interfere with the kit’s reagents and allowed us to achieve accurate detection of IL-12, thus improving the QC recovery to acceptable levels. This simple modification enabled us to successfully validate the assay according to regulatory guidance and meet the client’s sensitivity requirements.
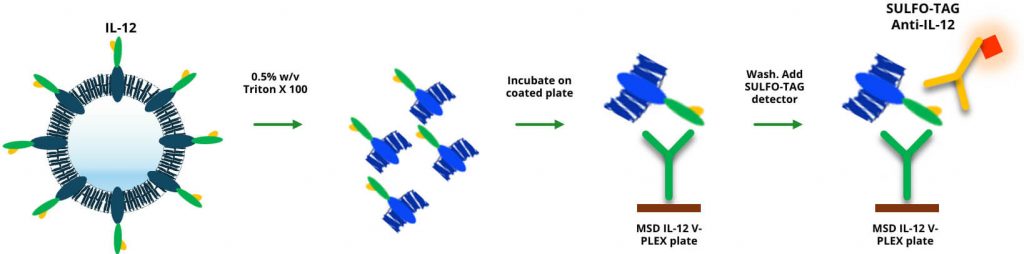
Supporting Illustration
The illustration depicts the dissociation of the endosome with Triton X-100 to make IL-12 more accessible for detection.
Driving Solutions in Bioanalytical Assay Development
This case study highlights the importance of a robust and adaptable approach in bioanalytical assay development, especially for novel drug modalities. By leveraging detailed intake processes and innovative problem-solving strategies, Immunologix Laboratories successfully overcame the challenges presented by Drug B, delivering reliable and accurate data for our clients.
This success underscores the importance of achieving scientific excellence through client collaboration, paving the way for more innovative and effective therapies to reach patients faster.
Have a Bioanalytical Assay Development Challenge?
Contact us today to learn how Immunologix Laboratories can support your drug development program with our comprehensive bioanalytical services.

Julia Allen, a dedicated Laboratory Manager who joined Immunologix as a Scientist in 2016 and specializes in pharmacokinetics and immunogenicity, leads the bioanalytical team by overseeing the execution of sample analyses, validations, and method developments.
Julia exemplifies the core values of a people manager at Immunologix by fostering a collaborative environment where her team members are encouraged to learn, contribute, and thrive. Her commitment to empowering others is evident in their progress and achievements.
